GHRP-2
Also known as: Growth Hormone Releasing Peptide 2, Pralmorelin, KP-102
GHRP-2 is a synthetic growth hormone secretagogue that stimulates GH release through the ghrelin receptor. It is considered one of the most potent GHRPs for GH stimulation.
GHRP-2 Overview & Molecular Profile
GHRP-2 (Pralmorelin) is a synthetic hexapeptide growth hormone secretagogue developed in the 1990s. It is considered one of the most potent members of the GHRP family for stimulating GH release. While it shares the mechanism of action with other GHRPs through ghrelin receptor activation, it has distinct characteristics including moderate effects on appetite and a favorable profile for GH research applications.
Mechanism of Action: Hormonal Signaling & Receptor Binding
GHRP-2 acts as a potent agonist at the ghrelin receptor (GHSR-1a), stimulating growth hormone release from the pituitary. It works synergistically with GHRH analogs through activation of different signaling pathways. GHRP-2 may also have some effects on cortisol and prolactin, though less pronounced than GHRP-6. Its appetite-stimulating effects are moderate compared to GHRP-6.
Research-Observed Effects
Potent GH Release
Research consistently demonstrates GHRP-2 (Pralmorelin) as one of the most potent growth hormone secretagogues available for research, producing robust GH elevations through high-affinity binding to the growth hormone secretagogue receptor (GHSR-1a) on pituitary somatotroph cells. Clinical studies document peak plasma GH concentrations of 30-100 ng/mL occurring approximately 15-30 minutes after subcutaneous administration, representing 8-20 fold increases above baseline levels depending on dosage and individual response characteristics. GHRP-2's potency for GH stimulation exceeds that of GHRP-6 on a microgram-per-microgram basis while producing less pronounced side effects on appetite and cortisol, making it particularly valuable for growth hormone deficiency treatment studies. The peptide maintains effectiveness in elderly subjects with diminished natural GH production, demonstrating consistent GH responses that decline only modestly with age compared to younger populations. Research protocols frequently combine GHRP-2 with GHRH analogs such as CJC-1295 or Sermorelin for synergistic growth hormone amplification, with studies showing 2-3 fold greater GH release compared to either compound alone.
IGF-1 Elevation
Studies demonstrate significant and sustained elevation of insulin-like growth factor 1 (IGF-1) following GHRP-2-induced growth hormone release, with effects persisting beyond the acute GH peak due to the downstream nature of IGF-1 production in the liver. Research shows IGF-1 increases of 25-75% above baseline within 7-14 days of consistent GHRP-2 administration, with levels stabilizing at elevated plateaus during continued treatment protocols. The IGF-1 elevation mediates many of GHRP-2's anabolic effects including enhanced protein synthesis, improved nitrogen retention, and accelerated muscle recovery optimization following resistance exercise in research models. Studies in growth hormone deficiency models demonstrate normalization of IGF-1 levels with appropriate GHRP-2 dosing, suggesting potential applications in growth hormone replacement therapy research. The relationship between GHRP-2 dosing and IGF-1 response provides researchers with a predictable biomarker for assessing treatment efficacy in various endocrine research applications including age-related hormone decline studies.
Moderate Appetite Effects
GHRP-2 produces significantly less appetite stimulation compared to GHRP-6, typically causing mild hunger increases in approximately 30-40% of research subjects compared to the near-universal appetite effects observed with GHRP-6. This reduced ghrelin-mimetic activity at hypothalamic appetite centers makes GHRP-2 particularly suitable for metabolic research studies where hunger confounds would complicate data interpretation. Research indicates GHRP-2's modest appetite effects result from lower intrinsic activity at the orexigenic signaling pathways downstream of GHSR-1a activation compared to its robust GH-releasing potency. The balanced profile between GH stimulation and appetite modulation positions GHRP-2 as an ideal research tool for body composition studies, muscle protein synthesis research, and investigations where maintaining controlled feeding conditions is essential. Studies comparing GHRP family members consistently rank GHRP-2 between Ipamorelin (minimal appetite effects) and GHRP-6 (strong appetite effects) on the spectrum of hunger induction.
Sleep Research
Preliminary research indicates GHRP-2 may enhance slow-wave sleep (SWS) duration and quality, which is notable because this deep sleep phase naturally coincides with the largest nocturnal growth hormone secretion pulse. Studies measuring polysomnographic parameters show increased time spent in stage 3 and stage 4 sleep following evening GHRP-2 administration, with some research documenting 20-35% increases in slow-wave sleep compared to placebo conditions. The enhancement of sleep quality appears synergistic with GHRP-2's GH-releasing effects, as both natural and peptide-induced GH release predominantly occurs during slow-wave sleep periods. Research suggests potential applications in sleep disorder studies, age-related sleep quality decline investigation, and recovery optimization research where sleep quality directly impacts anabolic processes. These sleep effects have generated interest in GHRP-2 for insomnia research, sleep architecture studies in the elderly, and investigations into the bidirectional relationship between growth hormone and sleep quality.
Pharmacokinetics
Plasma Concentration Profile (GHRP-2 Pharmacokinetics)
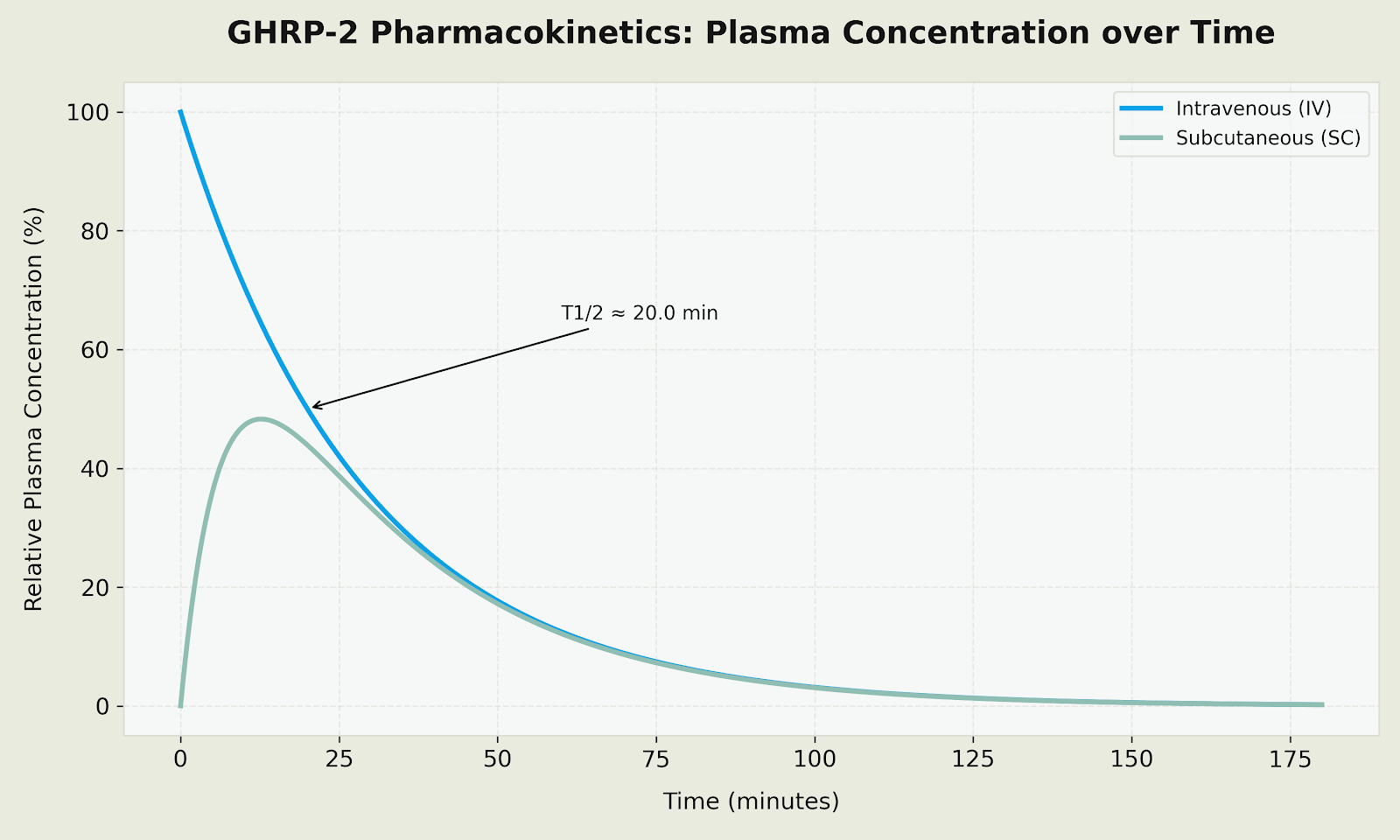
Figure: Relative plasma concentration profiles comparing IV (immediate peak with rapid decline) and SC (steep rise peaking at 15-20 minutes with T1/2 of approximately 20 min) administration routes for GHRP-2.
Kinetic Summary for GHRP-2
Research Dosing Information
Research protocols typically use 100-300 mcg administered 2-3 times daily. The peptide is often studied in combination with GHRH analogs.
Note: Dosing information is provided for research reference only and is based on published studies using research subjects. This is not a recommendation for any use.
Research Studies & References
Growth hormone-releasing peptide-2 infusion synchronizes growth hormone, thyrotrophin and prolactin release
Mericq V, Cassorla F, et al. (1996). European Journal of Endocrinology
This clinical study investigated how continuous GHRP-2 infusion affects the coordinated release of multiple pituitary hormones including growth hormone, thyroid-stimulating hormone (TSH), and prolactin in human subjects. Researchers administered GHRP-2 via intravenous infusion and measured hormone levels at frequent intervals to capture pulsatile secretion patterns and cross-hormone relationships. Results demonstrated that GHRP-2 produced robust, synchronized increases in GH release while causing modest elevations in TSH and prolactin through apparent pituitary cross-talk mechanisms. The study revealed that peak GH concentrations occurred within 30 minutes of infusion initiation, with levels 10-15 times above baseline, while TSH and prolactin effects were transient and returned to baseline within 2-3 hours. These findings contributed to understanding GHRP-2's pituitary effects profile and helped establish protocols for clinical applications in growth hormone deficiency assessment and treatment studies.
GHRP-2 as a GH-releasing factor in humans
Bowers CY, Momany FA, et al. (1999). Endocrinology and Metabolism Clinics of North America
This comprehensive clinical review examined GHRP-2's efficacy as a growth hormone releasing factor in human subjects across various age groups and clinical conditions, establishing its role as a research and diagnostic tool. The authors presented data from multiple clinical trials demonstrating GHRP-2's consistent ability to stimulate GH release in healthy volunteers, children with growth hormone deficiency, and elderly individuals with age-related GH decline. Dose-response analyses revealed optimal GH stimulation at doses of 100-300 mcg with diminishing returns at higher doses due to receptor saturation effects. The paper documented GHRP-2's superior GH-releasing potency compared to GHRP-6 while producing fewer side effects on appetite and cortisol levels. These findings established GHRP-2 as a valuable diagnostic agent for assessing pituitary GH reserve and laid groundwork for therapeutic applications in growth hormone deficiency treatment and anti-aging research protocols.
Effects of GHRP-2 on sleep and growth hormone release in normal adults
Moreno-Reyes R, Kerkhofs M, et al. (1998). European Journal of Endocrinology
This controlled study examined the relationship between GHRP-2 administration, sleep architecture changes, and nocturnal growth hormone release patterns in healthy adult volunteers using polysomnography and frequent blood sampling. Subjects received GHRP-2 or placebo before sleep onset while researchers monitored sleep stages and GH levels throughout the night. Results showed that evening GHRP-2 administration enhanced both slow-wave sleep duration by approximately 25% and the amplitude of nocturnal GH pulses by 40-60% compared to placebo. The study documented a positive correlation between slow-wave sleep enhancement and GH elevation, suggesting mechanistic links between peptide effects on sleep centers and somatotroph function. These findings have important implications for research into age-related sleep deterioration, the relationship between sleep quality and anabolic hormone production, and potential therapeutic applications of GHRP-2 in sleep disorder and recovery optimization research.
Comparative Research
Explore in-depth research analyses and comparative studies featuring GHRP-2.
Frequently Asked Questions
Ipamorelin
C38H49N9O5
Ipamorelin is a selective growth hormone secretagogue and ghrelin receptor agonist. It stimulates the release of growth hormone from the pituitary gland without significantly affecting cortisol or prolactin levels.
CJC-1295
C152H252N44O42
CJC-1295 is a synthetic analog of growth hormone releasing hormone (GHRH) with a Drug Affinity Complex that extends its half-life significantly compared to native GHRH.