BPC-157
Also known as: Body Protection Compound-157, Pentadecapeptide BPC 157
BPC-157 is a synthetic pentadecapeptide derived from a protective protein found in gastric juice. It has been extensively studied for its potential regenerative and protective properties in various tissue types.
BPC-157 Overview & Molecular Profile
BPC-157, or Body Protection Compound-157, is a 15 amino acid peptide chain that has been the subject of extensive research since its discovery. Originally isolated from human gastric juice, this synthetic peptide has demonstrated remarkable stability and bioactivity in numerous preclinical studies. Research has focused on its potential applications in tissue repair, wound healing, and protective effects against various forms of damage. The peptide is notable for its stability in human gastric juice and resistance to degradation.
Mechanism of Action: Gene Activation & Angiogenesis
BPC-157 is believed to work through multiple mechanisms, including upregulation of growth hormone receptors, modulation of nitric oxide synthesis, and interaction with the dopaminergic system. Research suggests it may promote angiogenesis (formation of new blood vessels) and accelerate the healing cascade. Studies indicate potential involvement with the FAK-paxillin pathway, which plays a role in cell adhesion and tissue repair. Additionally, BPC-157 may interact with the nitric oxide system to promote vascular function and tissue regeneration.
Research-Observed Effects
Tissue Repair & Regeneration
Preclinical studies have demonstrated remarkable acceleration of healing across multiple tissue types including skeletal muscle tears, tendon injuries, ligament damage, and bone fractures. Research published in peer-reviewed journals shows BPC-157 enhances collagen synthesis and promotes proper tissue remodeling through upregulation of growth hormone receptors in fibroblasts. Studies in animal models have documented faster recovery from muscle crush injuries, Achilles tendon transection, and medial collateral ligament damage. The peptide appears to promote formation of granulation tissue and accelerate the transition from inflammatory to proliferative healing phases. Research indicates potential applications in sports medicine research for studying muscle strain recovery, rotator cuff repair mechanisms, and connective tissue regeneration pathways.
Gastrointestinal Protection
Extensive research demonstrates powerful cytoprotective properties throughout the gastrointestinal tract, with studies showing protection against NSAID-induced gastric lesions, alcohol-induced stomach damage, and stress ulcers. BPC-157 has been investigated for inflammatory bowel disease mechanisms including ulcerative colitis and Crohn's disease models, showing reduced mucosal inflammation and accelerated intestinal healing. Research indicates the peptide may protect against esophageal damage, promote healing of intestinal anastomoses after surgery, and reduce liver damage from various toxins. Studies have documented effects on the gut-brain axis and potential benefits for intestinal permeability issues often referred to as leaky gut syndrome. The peptide's remarkable stability in gastric juice allows for oral administration in research settings, making it unique among therapeutic peptides.
Anti-inflammatory Activity
Research demonstrates significant modulation of inflammatory pathways including reduction of pro-inflammatory cytokines such as TNF-alpha, IL-1 beta, and IL-6 in multiple tissue models. BPC-157 appears to counteract oxidative stress damage through enhancement of antioxidant enzyme systems and reduction of reactive oxygen species in damaged tissues. Studies show the peptide may reduce inflammation associated with adjuvant-induced arthritis, periodontitis, and various autoimmune condition models. The anti-inflammatory effects appear synergistic with tissue healing properties, creating an optimal environment for regeneration. Research indicates potential applications in studying chronic inflammatory conditions, post-surgical inflammation reduction, and inflammation-related pain mechanisms.
Wound Healing Acceleration
Multiple controlled studies demonstrate significantly enhanced wound closure rates, with research showing up to 2-3 times faster healing in various wound models compared to controls. BPC-157 promotes robust granulation tissue formation, accelerated re-epithelialization, and improved tensile strength of healing wounds. Studies document enhanced angiogenesis with increased blood vessel formation in wound beds, providing better oxygen and nutrient delivery to healing tissues. Research in burn wound models, diabetic wound models, and surgical incision healing shows consistent acceleration of all phases of wound repair. The peptide has been studied for potential applications in chronic wound management studies, skin graft survival enhancement, and post-operative incision healing optimization.
Neuroprotective Effects
Preliminary research indicates significant protective effects on neural tissue including protection against chemotherapy-induced peripheral neuropathy and traumatic brain injury models. Studies suggest BPC-157 may promote peripheral nerve regeneration after crush injuries, with documented improvements in nerve fiber density and functional recovery. Research in dopaminergic system models shows potential relevance to Parkinson's disease mechanisms, with studies documenting protection against MPTP and other neurotoxic agents. The peptide appears to interact with nitric oxide pathways in the central nervous system, potentially influencing neurotransmitter balance and synaptic function. Emerging research explores applications in spinal cord injury repair, stroke recovery mechanisms, and protection against neurodegenerative processes.
Organoprotection
Research demonstrates significant protective effects across multiple organ systems in preclinical models. Cardiac studies show elimination of arrhythmias in rat models and protection against ischemia-reperfusion injury. Hepatoprotective effects include reduced liver damage from alcohol and various toxins. Renal protection has been observed in models of acute kidney injury, with improved function markers and reduced tissue damage. Pulmonary studies indicate protection against lung injury models. The peptide appears to maintain organ function under stress conditions through nitric oxide system modulation and vascular protection mechanisms.
The Pharmacokinetic Precision Gap
Researchers often cite estimated metabolic rates for BPC-157, but precise preclinical data provides a more nuanced understanding of its therapeutic behavior. Understanding the interplay between its rapid clearance and its prolonged biological effect is essential for interpreting its unique research applications.
Systemic Half-Life: The 15.2-Minute Reality
Formal pharmacokinetic studies have established that BPC-157 is cleared from systemic circulation with remarkable speed.
- •Mean Elimination Half-Life (t½): In rats, the average elimination half-life after a single intravenous (IV) administration is 15.2 minutes.
- •Prototype Clearance: The prototype drug typically becomes undetectable in plasma within 4 hours post-administration.
- •Metabolic Fate: BPC-157 is primarily metabolized in the liver, where it is broken down into smaller peptide fragments and individual amino acids that enter normal metabolic pathways.
The Gene Expression Paradox
Despite a systemic presence measured in minutes, BPC-157 exerts regenerative effects that persist for weeks. This is often described as the Gene Expression Paradox, a phenomenon where a short-lived peptide initiates a permanent healing cascade.
- •Rapid Gene Activation: Within the first hour of administration, BPC-157 triggers a significant burst of gene expression.
- •Tendon-Specific Upregulation: Microarray analysis reveals that BPC-157 significantly increases the expression of the Growth Hormone Receptor (GHR) in tendon fibroblasts.
- •Biological Durability: The peptide's immediate interaction with transcription factors like c-Fos, c-Jun, and Egr-1 allows for angiogenic and anti-inflammatory processes that far outlast the physical presence of the molecule.
Plasma Concentration Profile (BPC-157 Pharmacokinetics)
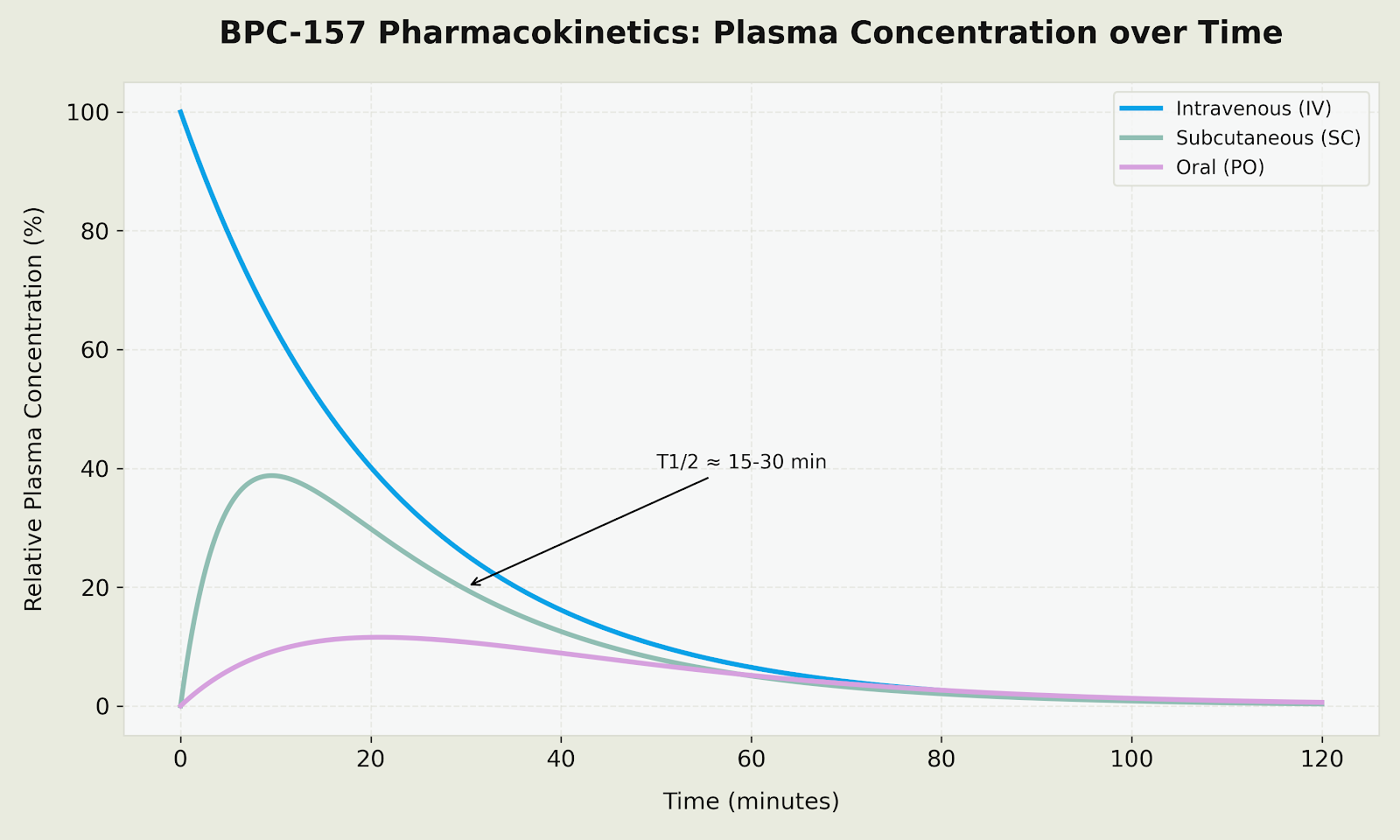
Figure: Relative plasma concentration profiles comparing IV (rapid distribution), SC (gradual absorption with T1/2 of 15-30 min), and oral (sustained release due to high gastric stability) administration routes.
The Precision of BPC-157 Angiogenesis
While many compounds can force the growth of new blood vessels (angiogenesis), they often do so in a disorganized way that can lead to leaky vessels or unwanted tissue growth. BPC-157 is distinguished in research for its ability to promote organized, stable vessel formation through a series of molecular safety checks.
The Master Switch and the Brake (Egr-1 / NAB2 Loop)
In preclinical models, BPC-157 doesn't just push the on switch for healing; it manages the entire process like a thermostat.
- •The Master Switch: It rapidly activates a gene called Egr-1, which acts as a master controller to start building the proteins and vessels needed to close a wound.
- •The Internal Brake: Simultaneously, BPC-157 triggers NAB2, a natural protein that acts as a brake on Egr-1.
- •Organized Repair: By turning on both the switch and the brake at the same time, BPC-157 ensures the body produces enough new vessels to repair the injury without allowing the chaotic, excessive growth often seen in chronic inflammation or tumor environments.
Maintaining Vascular Balance (The NO System)
Beyond direct growth, BPC-157 acts as a sophisticated moderator for Nitric Oxide (NO), the gas the body uses to relax blood vessels and direct blood flow to an injury.
- •Unlocking the System: In its resting state, the enzyme that produces nitric oxide is often locked and inactive. BPC-157 triggers a specific chemical signal (Src-Caveolin-1 phosphorylation) that unlocks this enzyme, allowing a controlled release of nitric oxide to start the healing process.
- •Homeostatic Control: Because this works on a regulatory level, BPC-157 is modulatory. This means it can help the body if there is too little nitric oxide, but it also has the potential to step in if levels become dangerously high. This balancing act helps maintain vascular stability and prevents the system-wide collapse that can occur when nitric oxide levels are out of sync.
Why This Matters for Research
This controlled approach to angiogenesis is why BPC-157 is often studied in sensitive areas like the eye or the gut. For example, while other growth factors might cause cloudy vision by growing vessels where they don't belong, research shows BPC-157 can promote healing in the eye while actively maintaining corneal transparency by opposing pathological vessel growth.
Research Dosing Information
In published research studies, BPC-157 has been administered at various dosages depending on the research model. Animal studies have typically used doses ranging from 10 mcg/kg to 10 mg/kg body weight. Administration routes in research include subcutaneous, intramuscular, intraperitoneal, and oral. Researchers should consult original study protocols for specific experimental conditions.
Note: Dosing information is provided for research reference only and is based on published studies using research subjects. This is not a recommendation for any use.
Research Studies & References
Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease
Sikiric P, Seiwerth S, et al. (2011). Current Pharmaceutical Design
This comprehensive review analyzes over two decades of BPC-157 research focusing specifically on inflammatory bowel disease applications including ulcerative colitis and Crohn's disease models. The authors present extensive evidence demonstrating the peptide's cytoprotective and mucosal healing properties across multiple animal models of intestinal inflammation. Key findings include significant reduction in inflammatory lesions, accelerated mucosal regeneration, and restoration of intestinal barrier function. The review also examines BPC-157's unique mechanism involving nitric oxide system modulation, growth factor interaction, and anti-inflammatory pathway activation. The authors conclude that BPC-157 represents a promising therapeutic research candidate for gastrointestinal conditions based on its consistent efficacy across diverse experimental models and its remarkable safety profile in preclinical testing.
Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts
Chang CH, Tsai WC, et al. (2014). Molecules
This mechanistic study investigates the molecular pathways through which BPC-157 promotes tendon healing, specifically focusing on growth hormone receptor (GHR) expression in human tendon fibroblasts. Using cell culture models and molecular analysis techniques including RT-PCR and Western blotting, researchers demonstrated that BPC-157 treatment significantly upregulates GHR expression in a dose-dependent manner. The study reveals that enhanced GHR expression leads to increased sensitivity to growth hormone signaling, promoting fibroblast proliferation, collagen synthesis, and extracellular matrix production. These findings provide crucial mechanistic insight into how BPC-157 accelerates tendon repair and have significant implications for understanding tendinopathy treatment mechanisms, rotator cuff tear healing, and Achilles tendon injury recovery pathways.
BPC 157 and standard angiogenic growth factors: Effect on angiogenesis and wound healing
Seveljevic-Jaran D, Cuzic S, et al. (2018). Journal of Physiology and Pharmacology
This comparative study examines BPC-157's angiogenic properties alongside standard growth factors including VEGF (Vascular Endothelial Growth Factor) and bFGF (basic Fibroblast Growth Factor) in established wound healing models. The research demonstrates that BPC-157 promotes new blood vessel formation through distinct mechanisms while achieving comparable or superior results to traditional angiogenic factors. Microscopic analysis revealed increased capillary density, improved vessel maturation, and enhanced blood flow in BPC-157 treated wounds. The study documented accelerated wound closure rates, improved granulation tissue quality, and better cosmetic outcomes. Importantly, researchers found that BPC-157's angiogenic effects were achieved without the tumor-promoting concerns associated with some standard growth factors, suggesting a favorable safety profile for wound healing research applications.
Muscle healing and BPC 157: A comprehensive review of preclinical evidence
Sikiric P, et al. (2019). Journal of Orthopaedic Surgery and Research
This extensive review synthesizes findings from numerous preclinical studies examining BPC-157's effects on skeletal muscle injury repair including muscle tears, crush injuries, and denervation-induced atrophy models. The compiled evidence demonstrates consistent acceleration of muscle fiber regeneration, reduced scar tissue formation, and improved functional recovery across diverse experimental conditions. Mechanistic analysis reveals BPC-157 promotes satellite cell activation, myoblast proliferation and differentiation, and proper alignment of regenerating muscle fibers. The review also examines BPC-157's effects on muscle vascularization, inflammation resolution, and neuromuscular junction reformation. Authors highlight the peptide's potential significance for sports medicine research, post-surgical rehabilitation studies, and understanding muscle regeneration biology in conditions such as muscular dystrophies and sarcopenia.
Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway
Hsieh MJ, Lee CH, Chueh HY, Chang GJ, Huang HY, Lin Y, Pang JHS (2020). Scientific Reports
This study demonstrates BPC-157's concentration-dependent vasodilation effect in isolated rat aorta through endothelium-dependent mechanisms. The research reveals that BPC-157 induces nitric oxide generation via activation of the Src-Caveolin-1-eNOS signaling pathway. Key findings include enhanced phosphorylation of Src, Caveolin-1, and eNOS, with the vasodilation effect being abolished by nitric oxide inhibitors. The study provides mechanistic insight into how BPC-157 modulates vascular tone and promotes endothelial cell migration, supporting its role in angiogenesis and cardiovascular protection.
Stable Gastric Pentadecapeptide BPC 157 and Wound Healing
Seiwerth S, Milavic M, Vukojevic J, Gojkovic S, Krezic I, et al. (2021). Frontiers in Pharmacology
This comprehensive review examines BPC-157's wound healing capabilities across multiple tissue types including skin incisions, deep burns, diabetic ulcers, and alkali burns. The authors demonstrate that BPC-157 simultaneously heals cutaneous and internal wounds in various fistula models. The peptide's healing mechanism involves resolution of vessel constriction, platelet plug formation, and fibrin stabilization. Notably, BPC-157 counteracts both arterial and venous thrombosis while promoting vessel circumvention in ischemia-reperfusion scenarios. The review also documents rapid gene expression changes in skin wounds that parallel healing observed in gastrointestinal tract, tendon, ligament, muscle, bone, nerve, and blood vessels.
Comparative Research
Explore in-depth research analyses and comparative studies featuring BPC-157.
Technical Deep Dives
BPC-157 Stability: Storage, Reconstitution, and Degradation Factors in Research Applications
Lyophilized BPC-157 is highly stable when stored at -20°C, maintaining integrity for years. Once reconstituted in bacteriostatic water (BAC water), BPC-157 should be refrigerated at 2-8°C and used wit...
BPC-157 and VEGF: Understanding the Angiogenesis-Mediated Healing Mechanism
BPC-157 promotes tissue healing primarily through potent angiogenic activity—the formation of new blood vessels. It significantly upregulates VEGF (vascular endothelial growth factor) expression, stim...
Comparative Clinical Analysis
BPC-157 vs TB-500: Comprehensive Comparison of Recovery and Healing Peptides for Research
BPC-157 and TB-500 are two peptides extensively studied in preclinical research for their potential influence on tissue repair processes, with complementary but distinct mechanisms. BPC-157, a stable ...
GHK-Cu vs BPC-157: Copper Peptide vs Gastric Peptide Comparison for Tissue Repair Research
GHK-Cu and BPC-157 are two of the most researched peptides for tissue repair, with distinct but potentially complementary mechanisms. GHK-Cu, a naturally occurring copper-binding tripeptide, excels in...
BPC-157 vs KPV: Gastric Pentadecapeptide vs Anti-Inflammatory Tripeptide Comparison
BPC-157 and KPV represent different approaches to tissue healing and inflammation control, with overlapping applications in gastrointestinal research. BPC-157, a stable gastric pentadecapeptide, promo...
Frequently Asked Questions
TB-500
C212H350N56O78S
TB-500 is a synthetic version of the naturally occurring peptide Thymosin Beta-4, which plays a crucial role in tissue repair, cell migration, and blood vessel formation in the body.
GHK-Cu
C14H23CuN6O4
GHK-Cu is a naturally occurring copper complex of the tripeptide glycyl-L-histidyl-L-lysine. It has been extensively studied for wound healing, skin rejuvenation, and tissue remodeling.